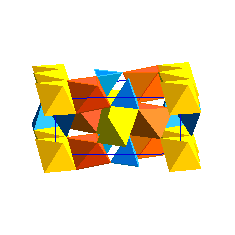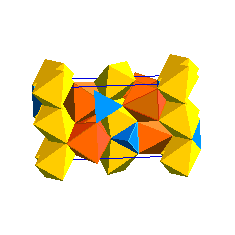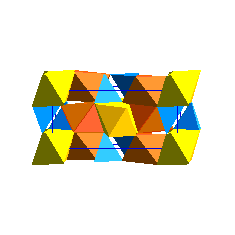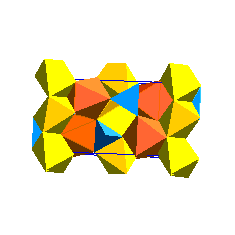|
What you need to learn:
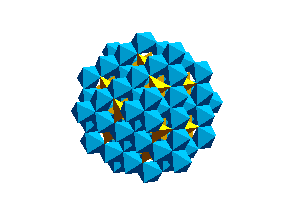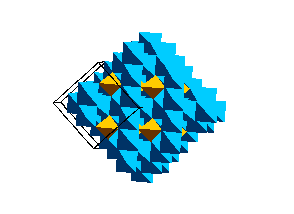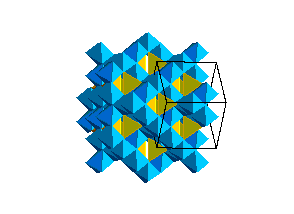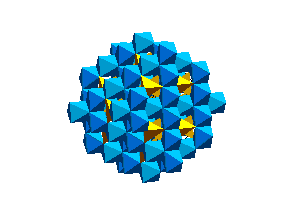
Olivine Structure:Below is a unit cell of the olivine structure.
Solid solution and configurational entropyThe cation disorder over the M1 and M2 sites gives olivine a slightly higher stability at elevated temperatures because of the increased configurational entropy associated with disorder. There are two sites for Mg and Fe in olivine, M1 and M2. The configurations Fe(M1)Mg(M2) and Fe(M2)Mg(M1) have almost the same energy. Both the M1 and M2 sites will have equal amounts of Mg or Fe. Olivine is a good example of a nearly ideal solid solution. Now, let XM1 be the mole fraction of Mg in the M1 site; let XM2 be the mole fraction of Mg in the M2 site. The configurational entropy will be S = [XM1lnXM1 + (1-XM1)ln(XM1) + XM2lnXM2 +(1-XM2)ln(1-XM2)] Since there is no partitioning between the M1 and M2 sites, XM2 = XM1 = X so that S = 2R[XlnX+(1-X)ln(1-X)]
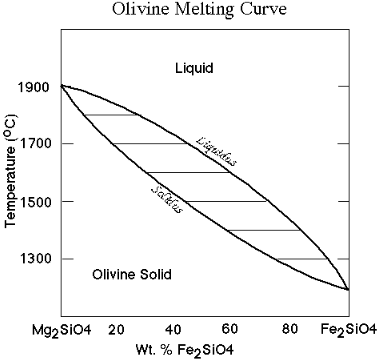
Effect of solid solution on melting
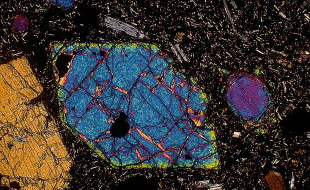
Optical Properties of OlivineOlivine can be recognized by its
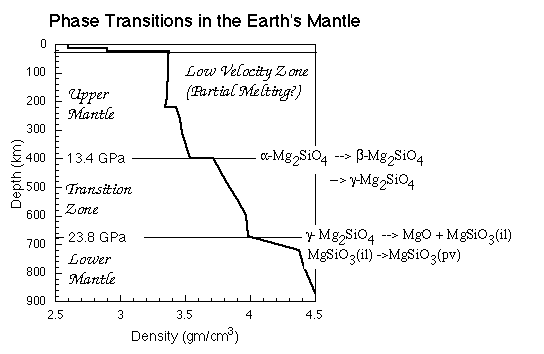
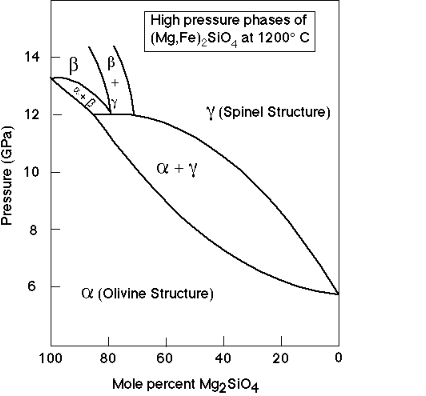
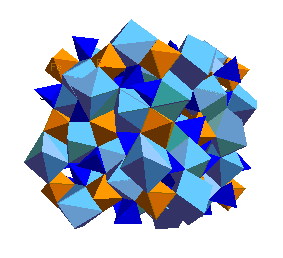
High-Pressure Phase Transitions of OlivineUnder the pressure conditions of the Earth's mantle, olivine undergoes several phase transitions to denser structures. These phase transitions give rise to several important seismic discontinuities. Indeed, the transition from the olivine to the spinel structure is responsible for deep focus earthquakes. It is probably worthwhile, both for this course and for others in the next two years, that you memorize the diagram below:
The spinel (ringwoodite) polymorph
WadsleyiteIntermediate between the olivine and spinel (ringwoodite) structure is a phase known as wadsleyite (beta (Mg,Fe)2SiO4 The wadsleyite (or beta-spinel) structure is currently of great interest because it is being invoked as a reservior of water (as chemically bound OH) in the Earth's mantle.
There is more polyhedral sharing in the wadsleyite and spinel structures than in the olivine structure. According to Paulings Rules, therefore, these structures should have a higher internal energy than the olivine structure. They form because at high pressure, phases with a lower volume (higher density) will have a lower free energy.
The pressure of the phase transitions to ringwoodite and wadsleyite varies with composition (Fe/Mg ratio). This has implications for the sharpness of these transitions in the Earth's mantle and is currently of research interest:
Garnets
Crystal ChemistryGeneral formula is X3Y2Z3O12 where X = Ca2+, Mg2+, Fe2+, Mn2+ (Dodecahedral Site) Y = Fe3+, Al3+, Cr3+ (Octahedral Site) Z = Si4+, Ti4+ (Tetrahedral Site) Nomenclature:
There is complete solid solution between pyrope-almandine and spessertine ("pyralspite") and also between grossular, andradite and uvarovite ("ugrandite"). The reason there is no solid solution between the "pyralspite" and "ugrandite" systems is because of the ionic radii differences between Ca and (Mg, Fe2+ or Mn2+).
Crystallographic and Optical Properties:Garnets are cubic. In thin section, garnets have high relief and are optically isotropic. They show no cleavage and have variable colours depending on the transition metal present. Rarely, they are slightly optically anistropic... Garnet ParagenesisGarnets occur in a variety of igneous and metamorphic environments:
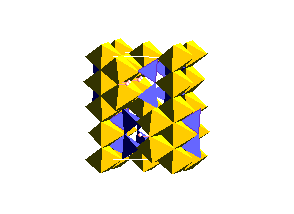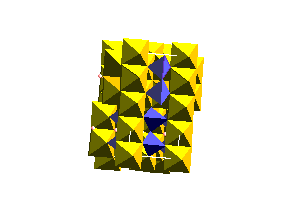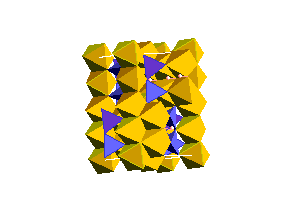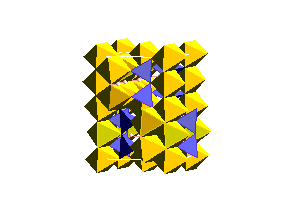
There is also a garnet structure of MgSiO3 at high pressure:
All Pages Copyright © GeoClassroom. All Rights Reserved. |