|
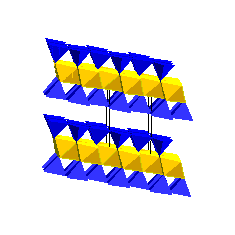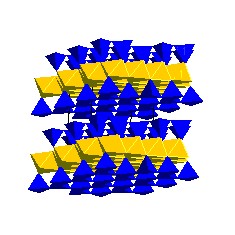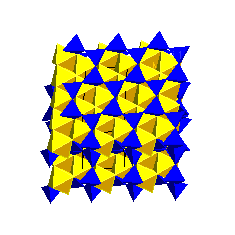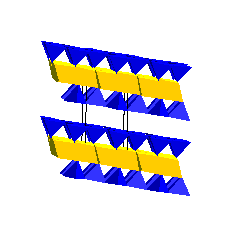
Mica GroupIf we start with a simple 2:1 phyllosilicate such as talc or pyrophyllite and replace some of the Si with Al, the charge on the 2:1 layer will decrease by -1 for every Si replaced. The negatively charged layers would now attract positive cations (e.g., Na, K, Ca) which can then hold the layers together. As far as rock-forming minerals are concerned, the most important example are the micas. Here is the structure of muscovite:
Mica CompositionsThe general formula of micas is A0-1Y2-3(Z4O10)(OH,F)2 where A are the interlayer cations (K+, Ca2+ and Na+), Y are the octahedral-layer cations (Fe, Mg and Al), and Z are the tetrahedral cations (Si and Al).
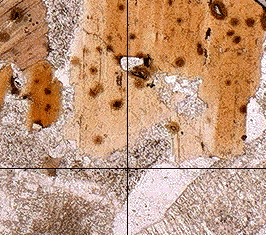
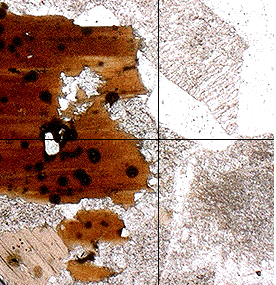
Optical Properties of MicasMicas are fairy easy to identify under the microscope. Biotite, for example, always shows a strong characteristic pleochroism:
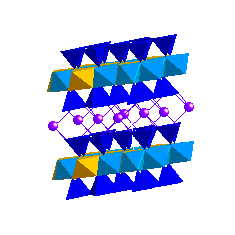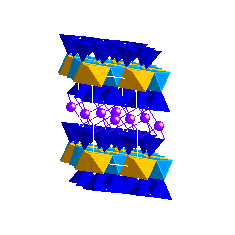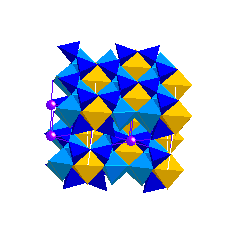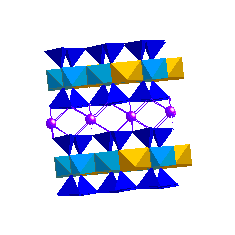
VermiculiteIntermediate between the smectites and micas is vermiculite. This phase is defined by its intermediate layer charge. An important feature of both smectites and vermiculites is their ability to exchange cations between the interlayer and a coexisting aqueous solution.
IlliteIllites may be thought of as potassium-deficient muscovites. Depending on how the interlayer charge deficiency is made up we can get illite, hydromuscovite or phengite:
Illites are the dominant clay minerals in shales and mudstones. The presence of interlayer K prevents H2O and organic molecules from entering the interlayer sites. Consequently, illites do not expand and have a low cation exchange capacity. Smectites transform to illites and also form complex illite-smectite mixed layer clays during diagenesis. Chlorite GroupChlorite consists of 2:1 layers that are held together by Mg(OH)2 sheets:
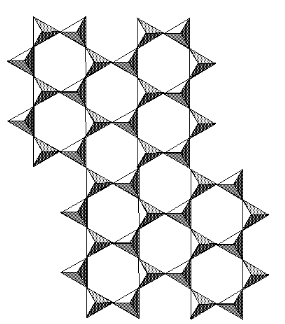
The Clay MineralsThe building blocks: octahedral and tetrahedral layers:The phyllosilicates are based on sheets of (Si,Al)O4 tetrahedra. Note below that there are two types of oxygens: bridging (shared by two tetrahedra) and apical (bonded to one tetrahedron). If the tetrahedral sites are occupied by Si(4+), then each bridging oxygen will receive a total Pauling bond strength sum of 2 x (4/4) = 2. The apical oxygens receive a net bond sum of 4/4 = 1. Hence, the apical oxygens need to be bonded to something else.
That something else is the octahedral layer made up of (Fe,Mg)O6 or AlO6 octahedra. We can derive a first-order picture of the phyllosilicates in terms of the tetrahedral and octahedral layers: 1:1 Phyllosilicate StructuresThe simplest phyllosilicates result from bonding one silicate layer to one octahedral layer. Because of this arrangement, we call these the 1:1 phyllosilicates. KaoliniteThe most important 1:1 phyllosilicate is the mineral kaolinite. The dioctahedral analogue is the serpentine mineral antigorite.
2:1 Phyllosilicate StructuresThe next group of phyllosilicates have one octahedral layer sandwiched between two tetrahedral layers. We call these the 2:1 structures. Talc and PyrophylliteThe simplest 2:1 structure is that of talc; the dioctahedral analogue is pyrophyllite.
Montmorillonite Group (Smectites) Clay Minerals
Montmorillonite and beidellite result from the alteration of volcanic ash to give bentonite clay deposits. In conditions of good drainage, Mg will be leached and kaolinite will form instead of montmorillonite. Smectites also result from the weathering and alteration of basic rocks. Nontronite results from the alteration of basaltic glass. All Pages Copyright © GeoClassroom. All Rights Reserved. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||